Copper catalysis

Copper-Catalyzed Functionalization of Enynes
Dherbassy, Q.; Manna, S.; Talbot, F. J. T.; Prasitwatcharakorn, P.; Perry, G. J. P.; Procter, D. J.
Chem. Sci. 2020, accepted manuscript
Dherbassy, Q.; Manna, S.; Talbot, F. J. T.; Prasitwatcharakorn, P.; Perry, G. J. P.; Procter, D. J.
Chem. Sci. 2020, accepted manuscript

Copper-Catalyzed Borylative Couplings with C–N
Electrophiles
Talbot, F. J. T.; Dherbassy, Q.; Manna, S.; Shi, C.;
Zhang, S.; Howell, G. P.; Perry, G. J. P.; Procter, D. J.
Angew. Chem. Int. Ed. 2020, 59, 2
Electrophiles
Talbot, F. J. T.; Dherbassy, Q.; Manna, S.; Shi, C.;
Zhang, S.; Howell, G. P.; Perry, G. J. P.; Procter, D. J.
Angew. Chem. Int. Ed. 2020, 59, 2

Copper-Catalyzed Functionalization of 1,3-Dienes: Hydrofunctionalization, Borofunctionalization and Difunctionalization
Perry, G. J. P.; Jia, T.; Procter, D. J.
ACS Catalysis 2020, 10, 1485
Perry, G. J. P.; Jia, T.; Procter, D. J.
ACS Catalysis 2020, 10, 1485
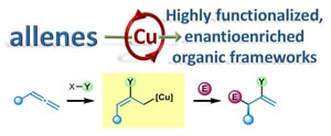
Enantioselective copper catalysed, direct functionalisation of allenes via allyl copper intermediates
Pulis, A. P.; Yeung, K.; Procter, D. J.
Chem. Sci. 2017, 8, 5240
Pulis, A. P.; Yeung, K.; Procter, D. J.
Chem. Sci. 2017, 8, 5240
Sulfonium mediated C-H functionalization

Radical C–C bond formation using sulfonium salts and light
Péter, Á.; Perry, G. J. P.; Procter, D. J.
Adv. Synth. Catal. 2020, accepted review article
• Invited contribution to the Special Issue 'Radical Chemistry and Organic Synthesis', edited by Armido Studer and Dennis Curran
• VIP paper
Péter, Á.; Perry, G. J. P.; Procter, D. J.
Adv. Synth. Catal. 2020, accepted review article
• Invited contribution to the Special Issue 'Radical Chemistry and Organic Synthesis', edited by Armido Studer and Dennis Curran
• VIP paper

C-H coupling reactions directed by sulfoxides: teaching an old functional group new tricks
Pulis, A. P.; Procter, D. J.
Angew. Chem. Int. Ed. 2016, 55, 9842
Pulis, A. P.; Procter, D. J.
Angew. Chem. Int. Ed. 2016, 55, 9842
Beyond the Pummerer Reaction: Recent Developments in Thionium Ion Chemistry
Smith, L. H. S.; Coote, S. C.; Sneddon, H. F.; Procter, D. J.
Angew. Chem. Int. Ed. 2010, 49, 5832
Smith, L. H. S.; Coote, S. C.; Sneddon, H. F.; Procter, D. J.
Angew. Chem. Int. Ed. 2010, 49, 5832
Samarium diiodide
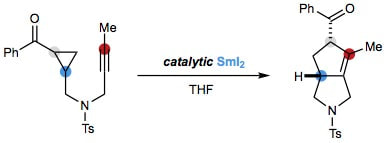
Cascades, Catalysis and Chiral Ligand Control with SmI2; The Rebirth of a Reagent
Péter, Á; Procter, D. J.
Chimia 2020, 74, 018
• Invited contribution to the Special Issue marking the Swiss Chemical Society/Syngenta Symposium: Welcome to the One-electron World
Péter, Á; Procter, D. J.
Chimia 2020, 74, 018
• Invited contribution to the Special Issue marking the Swiss Chemical Society/Syngenta Symposium: Welcome to the One-electron World
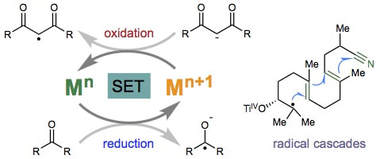
Radical cascade reactions triggered by single electron transfer
Plesniak, M. P.; Huang, H-M; Procter, D. J. Nat. Rev. Chem. 2017, 1, 0077 (2017). Issue cover |
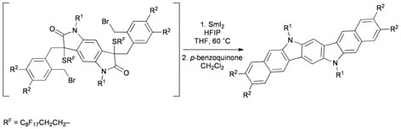
Overcoming synthetic challenges in target synthesis using SmI2: recent advances
Just-Baringo, X.; Yalavac, I.; Procter, D. J.
Organomet. Chem. 2016, 40, 1
Just-Baringo, X.; Yalavac, I.; Procter, D. J.
Organomet. Chem. 2016, 40, 1
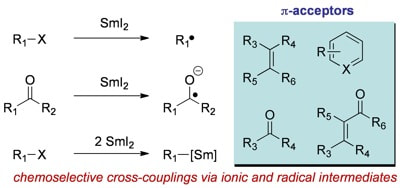
Cross-Coupling Reactions Using Samarium(II) Iodide
Szostak, M.; Fazakerley, N. J.; Parmar, D.; Procter, D. J.
Chem. Rev. 2014, 114, 5959
Szostak, M.; Fazakerley, N. J.; Parmar, D.; Procter, D. J.
Chem. Rev. 2014, 114, 5959
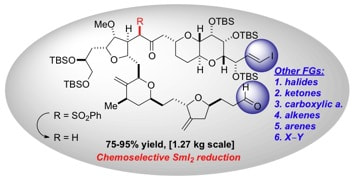
Recent advances in the chemoselective reduction of functional groups mediated by samarium(II) iodide: a single electron transfer approach
Szostak, M.; Spain, M.; Procter, D. J.
Chem. Soc. Rev. 2013, 42, 9155
Szostak, M.; Spain, M.; Procter, D. J.
Chem. Soc. Rev. 2013, 42, 9155

Organic Synthesis using Samarium Diiodide: A Practical Guide
Procter, D. J.; Flowers, R. A., II; Skrydstrup, T.
RSC Publishing 2009
Procter, D. J.; Flowers, R. A., II; Skrydstrup, T.
RSC Publishing 2009
Linker units cleaved by radical processes
Guazzelli, G.; Miller, M.; Procter, D. J.
In Linker Strategies In Solid–Phase Organic Synthesis, Ed. Peter J. H. Scott, Wiley, 2009, Chapter 15, 419.
Invited contribution
Guazzelli, G.; Miller, M.; Procter, D. J.
In Linker Strategies In Solid–Phase Organic Synthesis, Ed. Peter J. H. Scott, Wiley, 2009, Chapter 15, 419.
Invited contribution
Samarium enolates and their application in organic synthesis
Rudkin, I. M.; Miller, L. C.; Procter, D. J.
Organomet. Chem. 2008, 34, 19
Rudkin, I. M.; Miller, L. C.; Procter, D. J.
Organomet. Chem. 2008, 34, 19
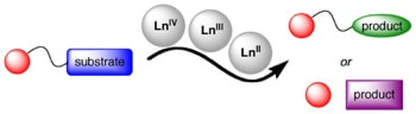
Lanthanide Reagents in Solid Phase Synthesis
Sloan, L. A.; Procter, D. J.
Chem. Soc. Rev. 2006, 35, 1221
Sloan, L. A.; Procter, D. J.
Chem. Soc. Rev. 2006, 35, 1221
Radical Chemistry on Solid Support
McGhee, A. M.; Procter, D. J.
Top. Curr. Chem. 2006, 264, 93
Invited contribution to a special issue on radical chemistry
McGhee, A. M.; Procter, D. J.
Top. Curr. Chem. 2006, 264, 93
Invited contribution to a special issue on radical chemistry
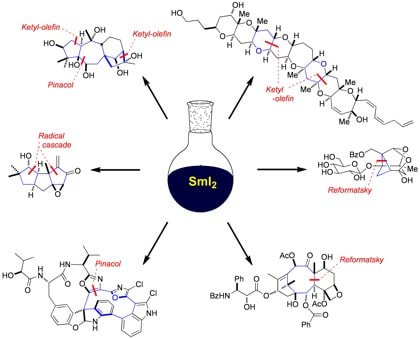
Samarium(II) iodide-mediated cyclizations in natural product synthesis
Edmonds, D. J.; Johnston, D.; Procter, D. J.
Chem. Rev. 2004, 104, 3371
Edmonds, D. J.; Johnston, D.; Procter, D. J.
Chem. Rev. 2004, 104, 3371
