Click on the reference to be taken to the article
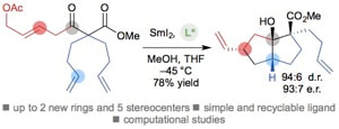
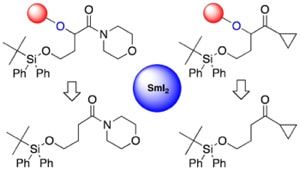
Enantioselective cyclizations and cyclization cascades of samarium ketyl radicals
Kern, N.; Plesniak, M. P.; McDouall, J. J. W.; Procter, D. J.
Nat. Chem. 2017, in press
Kern, N.; Plesniak, M. P.; McDouall, J. J. W.; Procter, D. J.
Nat. Chem. 2017, in press

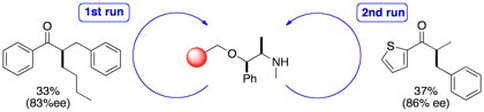
Enantioselective copper catalysed, direct functionalisation of allenes via allyl copper intermediates
Pulis, A. P.; Yeung, K.; Procter, D. J.
Chem. Sci. 2017, 8, 5240
Pulis, A. P.; Yeung, K.; Procter, D. J.
Chem. Sci. 2017, 8, 5240
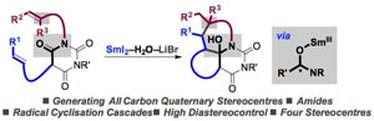
Selective construction of quaternary stereocentres in radical cyclisation cascades triggered by electron-transfer reduction of amide-type carbonyls
Huang, H-M.; Bonilla, P.; Procter, D. J.
Org. Biomol. Chem. 2017, 15, 4159
Invited contribution to themed issue on Polycyclizations in Synthesis and Biosynthesis
Huang, H-M.; Bonilla, P.; Procter, D. J.
Org. Biomol. Chem. 2017, 15, 4159
Invited contribution to themed issue on Polycyclizations in Synthesis and Biosynthesis
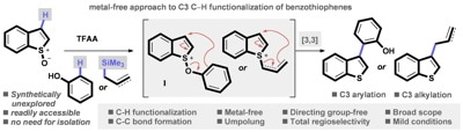
Regioselective synthesis of C3 alkylated and arylated benzothiophenes
Shrives, H. J.; Fernández-Salas, J. A.; Hedtke, C.; Pulis, A. P.; Procter, D. J.
Nat. Commun. 2017, 8, Article number: 14801
Shrives, H. J.; Fernández-Salas, J. A.; Hedtke, C.; Pulis, A. P.; Procter, D. J.
Nat. Commun. 2017, 8, Article number: 14801

Dearomatizing radical cyclizations and cyclization cascades triggered by electron-transfer reduction of amide-type carbonyls
Huang, H-M.; Procter, D. J.
J. Am. Chem. Soc. 2017, 139, 1661
Huang, H-M.; Procter, D. J.
J. Am. Chem. Soc. 2017, 139, 1661
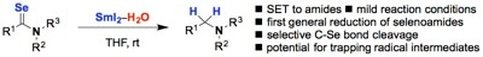
Reduction of selenoamides to amines using SmI2-H2O
Thurow, S.; Lenardao, E. J.; Just-Baringo, X.; Procter, D. J.
Org. Lett. 2017, 19, 50
Thurow, S.; Lenardao, E. J.; Just-Baringo, X.; Procter, D. J.
Org. Lett. 2017, 19, 50
Author profile: D. J. Procter
Angew. Chem. Int. Ed. 2017, 56, 5152
Angew. Chem. Int. Ed. 2017, 56, 5152
2016
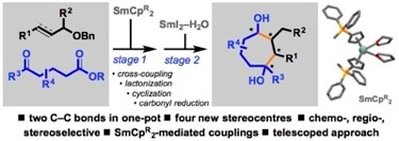
SmCp2-mediated cross-couplings of allyl and propargyl ethers with ketoesters and a telescoped approach to cycloheptanols
Plesniak, M. P.; Just-Baringo, X.; Ortu, F.; Mills, D. P.; Procter, D. J.
Chem. Commun. 2016, 52, 13503
Plesniak, M. P.; Just-Baringo, X.; Ortu, F.; Mills, D. P.; Procter, D. J.
Chem. Commun. 2016, 52, 13503
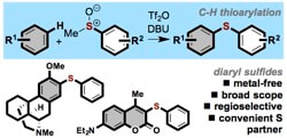
Metal-free C–H thioarylation of arenes using sulfoxides : A direct, general diaryl sulfide synthesis
Fernández-Salas, J. A.; Pulis, A. P.; Procter, D. J.
Chem. Commun. 2016, 52, 12364
Fernández-Salas, J. A.; Pulis, A. P.; Procter, D. J.
Chem. Commun. 2016, 52, 12364
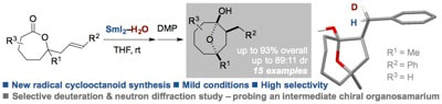
Selective synthesis of cyclooctanoids by radical cyclization of seven-membered lactones: Neutron diffraction study of the stereoselective deuteration of a chiral organosamarium intermediate
Just-Baringo, X.; Clark, J.; Gutmann, M. J.; Procter, D. J.
Angew. Chem. Int. Ed. 2016, 55, 12499
Just-Baringo, X.; Clark, J.; Gutmann, M. J.; Procter, D. J.
Angew. Chem. Int. Ed. 2016, 55, 12499
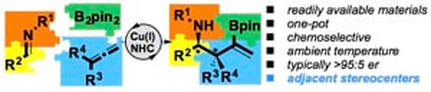
Enantioselective generation of adjacent stereocenters in a copper-catalyzed three-component coupling of imines, allenes and diboranes
Yeung, K.; Ruscoe, R. E.; Rae, J.; Pulis, A. P.; Procter, D. J.
Angew. Chem. Int. Ed. 2016, 55, 11912
Yeung, K.; Ruscoe, R. E.; Rae, J.; Pulis, A. P.; Procter, D. J.
Angew. Chem. Int. Ed. 2016, 55, 11912
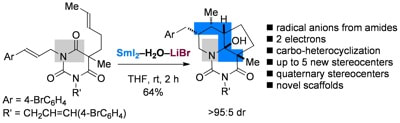
Radical-radical cyclization cascades of barbiturates triggered by electron-transfer reduction of amide-type carbonyls
Huang, H.; Procter, D. J.
J. Am. Chem. Soc. 2016, 138, 7770
Huang, H.; Procter, D. J.
J. Am. Chem. Soc. 2016, 138, 7770
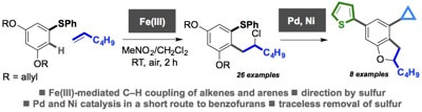
Iron-mediated oxidative C-H coupling of arenes and alkenes directed by sulfur: an expedient route to dihydrobenzofurans
Cavanagh, C. W.; Aukland, M. H. Aukland, Q. Laurent, A. Hennessy, Procter, D. J.
Org. Biomol. Chem. 2016, 14, 5286
Invited contribution to themed issue on Contemporary Synthetic Chemistry in Drug Discovery
Cavanagh, C. W.; Aukland, M. H. Aukland, Q. Laurent, A. Hennessy, Procter, D. J.
Org. Biomol. Chem. 2016, 14, 5286
Invited contribution to themed issue on Contemporary Synthetic Chemistry in Drug Discovery

C-H coupling reactions directed by sulfoxides: teaching an old functional group new tricks
Pulis, A. P.; Procter, D. J.
Angew. Chem. Int. Ed. 2016, 55, 9842.
Pulis, A. P.; Procter, D. J.
Angew. Chem. Int. Ed. 2016, 55, 9842.
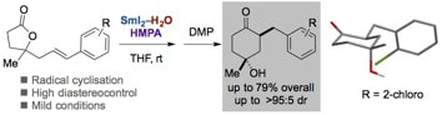
Highly selective SmI2-H2O-promoted radical cyclisation of five-membered lactones
Just-Baringo, X.; Morrill, C.; Procter, D. J.
Tetrahedron 2016, 72, 7691.
Invited contribution to Tetrahedron Symposium in Print, "Organic Chemistry - more radical than ever before"
Just-Baringo, X.; Morrill, C.; Procter, D. J.
Tetrahedron 2016, 72, 7691.
Invited contribution to Tetrahedron Symposium in Print, "Organic Chemistry - more radical than ever before"
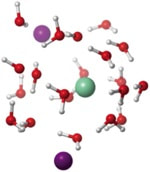
The role of H2O in the electron transfer-activation of substrates using SmI2: insights from DFT
Zhao, X.; Perrin, L.; Procter, D. J.; Maron, L.
Dalton Trans. 2016, 45, 3706
Zhao, X.; Perrin, L.; Procter, D. J.; Maron, L.
Dalton Trans. 2016, 45, 3706
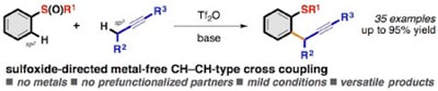
Metal-Free CH-CH-Type Cross-Coupling of Arenes and Alkynes Directed by a Multifunctional Sulfoxide group
Fernández-Salas, J. A.; Eberhart, A. J.; Procter, D. J.
J. Am. Chem. Soc. 2016, 138, 790
Fernández-Salas, J. A.; Eberhart, A. J.; Procter, D. J.
J. Am. Chem. Soc. 2016, 138, 790

Copper-catalyzed borylative cross-coupling of allenes and imines: Selective three-component assembly of branched homoallyl amines
Rae, J.; Yeung, K.; McDouall, J. J. W.; Procter, D. J.
Angew. Chem. Int. Ed. 2016, 55, 1102
Rae, J.; Yeung, K.; McDouall, J. J. W.; Procter, D. J.
Angew. Chem. Int. Ed. 2016, 55, 1102
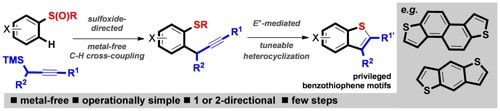
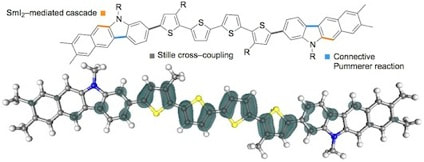
Sulfoxide-directed metal-free cross-couplings in the expedient synthesis of benzothiophene-based components of materials
Eberhart, A. J.; Shrives, H.; Zhang, Y.; Carrër, A.; Parry, A. V. S.; Tate, D. J.; Turner, M. L.; Procter, D. J.
Chem. Sci. 2016, 7, 1281
Eberhart, A. J.; Shrives, H.; Zhang, Y.; Carrër, A.; Parry, A. V. S.; Tate, D. J.; Turner, M. L.; Procter, D. J.
Chem. Sci. 2016, 7, 1281

Copper-catalyzed double additions and radical cyclization cascades in the re-engineering of the antibacterial pleuromutilin
Ruscoe, R. E.; Huang, H.; Flitsch, S.; Procter, D. J.
Chem. –Eur. J. 2016, 22, 116
Ruscoe, R. E.; Huang, H.; Flitsch, S.; Procter, D. J.
Chem. –Eur. J. 2016, 22, 116
2015
Overcoming synthetic challenges in target synthesis using SmI2: recent advances
Just-Baringo, X.; Yalavac, I.; Procter, D. J.
Organomet. Chem. 2016, 40, 1
Just-Baringo, X.; Yalavac, I.; Procter, D. J.
Organomet. Chem. 2016, 40, 1
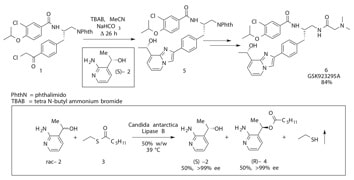
Cenp-E inhibitor GSK923295: novel synthetic route and use as a tool to generate aneuploidy
Bennett, A.; Bechi, B.; Tighe, A.; Thompson, S.; Procter, D. J.; Taylor, S. S. Oncotarget 2015, 6, 20921
Bennett, A.; Bechi, B.; Tighe, A.; Thompson, S.; Procter, D. J.; Taylor, S. S. Oncotarget 2015, 6, 20921

MYC Is a Major Determinant of Mitotic Cell Fate
Topham, C.; Tighe, A; Ly, P.; Bennett, A.; Sloss, O.; Nelson, L.; Ridgway, R. A.; Huels, D.; Littler, S.; Schandl, C.; Sun, Y.; Bechi, B.; Procter, D. J.; Sansom, O. J.; Cleveland, D. W.; Taylor, S. S.
Cancer Cell, 2015, 28, 129
Topham, C.; Tighe, A; Ly, P.; Bennett, A.; Sloss, O.; Nelson, L.; Ridgway, R. A.; Huels, D.; Littler, S.; Schandl, C.; Sun, Y.; Bechi, B.; Procter, D. J.; Sansom, O. J.; Cleveland, D. W.; Taylor, S. S.
Cancer Cell, 2015, 28, 129
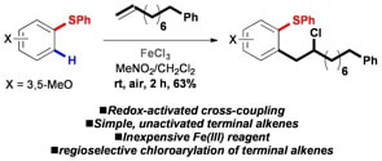
Iron-mediated C-H Coupling of Arenes and Unactivated Terminal Alkenes Directed by Sulfur
Cavanagh, C. W.; Aukland, M. H.; Hennessy, A.; Procter, D. J.
Chem. Commun. 2015, 51, 9272
Cavanagh, C. W.; Aukland, M. H.; Hennessy, A.; Procter, D. J.
Chem. Commun. 2015, 51, 9272
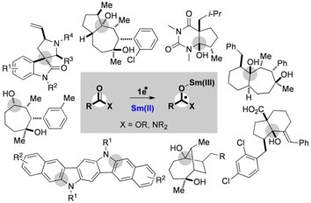
Sm(II)-Mediated Electron Transfer to Carboxylic Acid Derivatives: Development of Complexity Generating Cascades
Just-Baringo, X.; Procter, D. J.
Acc. Chem. Res. 2015, 48, 1263
Invited Account
Just-Baringo, X.; Procter, D. J.
Acc. Chem. Res. 2015, 48, 1263
Invited Account
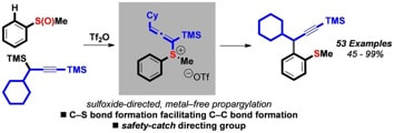
Sulfoxide-Directed Metal-Free ortho-Propargylation of Aromatics and Heteroaromatics
Eberhart, A. J.; Shrives, H. J.; Álvarez, E.; Carrër, A.; Zhang, Y.; Procter, D. J.
Chem. -Eur. J. 2015, 21, 7428
Hot paper, Cover art, Cover profile
Eberhart, A. J.; Shrives, H. J.; Álvarez, E.; Carrër, A.; Zhang, Y.; Procter, D. J.
Chem. -Eur. J. 2015, 21, 7428
Hot paper, Cover art, Cover profile
2014

Mechanism of SmI2/Amine/H2O-Promoted Chemoselective Reductions of Carboxylic Acid Derivatives (Esters, Acids, and Amides) to Alcohols
Szostak, M.; Spain, M.; Eberhart, A. J.; Procter,D. J.
J. Org. Chem. 2014, 79, 11988
Szostak, M.; Spain, M.; Eberhart, A. J.; Procter,D. J.
J. Org. Chem. 2014, 79, 11988
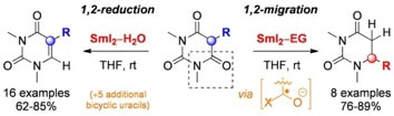
Switching between Reaction Pathways by an Alcohol Cosolvent Effect: SmI2−Ethylene Glycol vs SmI2−H2O Mediated Synthesis of Uracils
Szostak, M.; Spain, M.; Sautier, B.; Procter, D. J.
Org. Lett. 2014, 16, 5694
Szostak, M.; Spain, M.; Sautier, B.; Procter, D. J.
Org. Lett. 2014, 16, 5694
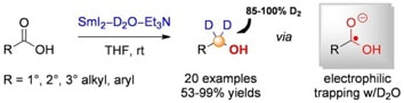
Selective Synthesis of α,α-Dideuterio Alcohols by the Reduction of Carboxylic Acids Using SmI2 and D2O as Deuterium Source under SET Conditions
Szostak, M.; Spain, M.; Procter, D. J.
Org. Lett. 2014, 16, 5052
Szostak, M.; Spain, M.; Procter, D. J.
Org. Lett. 2014, 16, 5052

SmI2-H2O-Mediated 5-exo/6-exo Lactone Radical Cyclisation Cascades
Yalavac, I.; Lyons, S. E.; Webb, M. R.; Procter, D. J.
Chem. Commun. 2014, 50, 12863
Invited contribution to web themed issue celebrating Richard Taylor's 65th birthday
Yalavac, I.; Lyons, S. E.; Webb, M. R.; Procter, D. J.
Chem. Commun. 2014, 50, 12863
Invited contribution to web themed issue celebrating Richard Taylor's 65th birthday
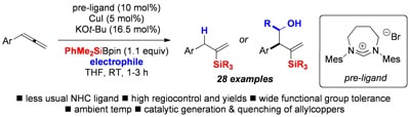
Cu(I)-NHC Catalyzed Silylation of Allenes: Diastereoselective Three Component Coupling with Aldehydes
Rae, J.; Hu, Y-C; Procter, D. J.
Chem. -Eur. J. 2014, 20, 13143
Highlighted in Synfacts 2014, 10(12), 1313
Rae, J.; Hu, Y-C; Procter, D. J.
Chem. -Eur. J. 2014, 20, 13143
Highlighted in Synfacts 2014, 10(12), 1313
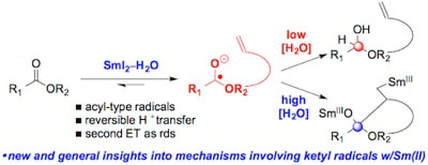
Ketyl-Type Radicals from Cyclic and Acyclic Esters are Stabilized by SmI2(H2O)n – The Role of SmI2(H2O)n in Post-Electron Transfer Steps
Szostak, M.; Spain, M.; Procter, D. J.
J. Am. Chem. Soc. 2014, 136, 8459
Szostak, M.; Spain, M.; Procter, D. J.
J. Am. Chem. Soc. 2014, 136, 8459
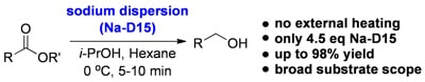
Evaluating a Sodium Dispersion Reagent for the Bouveault-Blanc Reduction of Esters
An, J.; Work, N. D.; Kenyon, C.; Procter, D. J.
J. Org. Chem. 2014, 79, 6743
An, J.; Work, N. D.; Kenyon, C.; Procter, D. J.
J. Org. Chem. 2014, 79, 6743
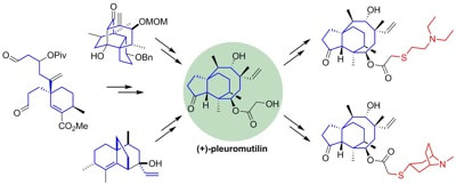
Synthesis and Synthetic Chemistry of Pleuromutilin
Fazakerley, N. J.; Procter, D. J.
Tetrahedron 2014, 70, 6911
Fazakerley, N. J.; Procter, D. J.
Tetrahedron 2014, 70, 6911
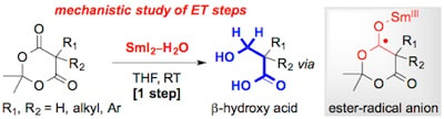
Mechanistic Investigation of the Selective Reduction of Meldrum's Acids to b-Hydroxy Acids using SmI2 and H2O
Szostak, M.; Lyons, S. E.; Spain, M.; Procter, D. J.
Chem. Commun. 2014, 50, 8391
Invited contribution to web themed issue on 'Non-Innocent Ligands'
Szostak, M.; Lyons, S. E.; Spain, M.; Procter, D. J.
Chem. Commun. 2014, 50, 8391
Invited contribution to web themed issue on 'Non-Innocent Ligands'
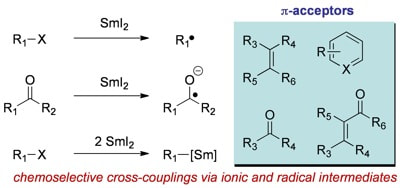
Cross-Coupling Reactions Using Samarium(II) Iodide
Szostak, M.; Fazakerley, N. J.; Parmar, D.; Procter, D. J.
Chem. Rev. 2014, 114, 5959
Szostak, M.; Fazakerley, N. J.; Parmar, D.; Procter, D. J.
Chem. Rev. 2014, 114, 5959

A Sm(II)-Mediated Cascade Approach to Dibenzoindolo[3,2-b]carbazoles: Synthesis and Evaluation
Levick, M. T.; Grace, I.; Dai, S-Y.; Kasch, N.; Muryn, C.; Lambert, C.; Turner, M. L.; Procter, D. J.
Org. Lett. 2014, 16, 2292
Highlighted in Synfacts 2014, 10(7), 0694
Levick, M. T.; Grace, I.; Dai, S-Y.; Kasch, N.; Muryn, C.; Lambert, C.; Turner, M. L.; Procter, D. J.
Org. Lett. 2014, 16, 2292
Highlighted in Synfacts 2014, 10(7), 0694
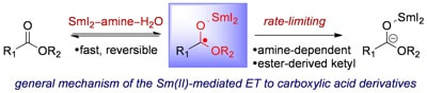
On the Role of Pre- and Post-Electron Transfer Steps in the SmI2/amine/H2O-Mediated Reduction of Esters: New Mechanistic Insights and Kinetic Studies
Szostak, M.; Spain, M.; Procter, D. J.
Chem. –Eur. J. 2014, 20, 4222.
Szostak, M.; Spain, M.; Procter, D. J.
Chem. –Eur. J. 2014, 20, 4222.
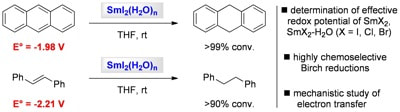
Determination of the Effective Redox Potentials of SmI2, SmBr2, SmCl2, and their Complexes with Water by Reduction of Aromatic Hydrocarbons. Reduction of Anthracene and Stilbene by Samarium(II) Iodide–Water Complex
Szostak, M.; Spain, M.; Procter, D. J.
J. Org. Chem. 2014, 79, 2522.
Szostak, M.; Spain, M.; Procter, D. J.
J. Org. Chem. 2014, 79, 2522.
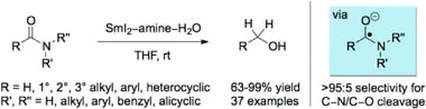
Highly Chemoselective Reduction of Amides (Primary, Secondary, Tertiary) to Alcohols using SmI2/Amine/H2O under Mild Conditions
Szostak, M.; Spain, M.; Eberhart, A. J.; Procter, D. J.
J. Am. Chem. Soc. 2014, 136, 2268
Highlighted in Synfacts 2014, 10(5), 0527
Szostak, M.; Spain, M.; Eberhart, A. J.; Procter, D. J.
J. Am. Chem. Soc. 2014, 136, 2268
Highlighted in Synfacts 2014, 10(5), 0527
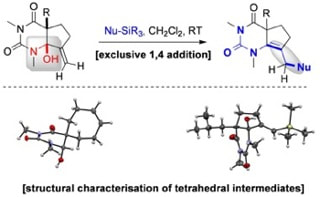
Structural analysis and reactivity of unusual tetrahedral intermediates enabled by SmI2-mediated reduction of barbituric acids: vinylogous N-acyliminium additions to α-hydroxy-N-acyl-carbamides
Szostak, M.; Sautier, B.; Procter, D. J.
Chem. Commun. 2014, 50, 2518
Szostak, M.; Sautier, B.; Procter, D. J.
Chem. Commun. 2014, 50, 2518
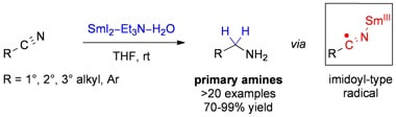
Electron Transfer Reduction of Nitriles Using SmI2–Et3N–H2O: Synthetic Utility and Mechanism
Szostak, M.; Sautier, B.; Spain, M.; Procter, D. J.
Org. Lett. 2014, 16, 1092.
Szostak, M.; Sautier, B.; Spain, M.; Procter, D. J.
Org. Lett. 2014, 16, 1092.
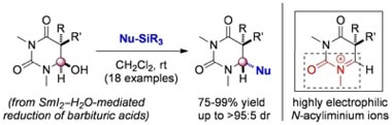
Stereoselective Capture of N-Acyliminium Ions Generated from α-Hydroxy-N-acylcarbamides: Direct Synthesis of Uracils from Barbituric Acids Enabled by SmI2 Reduction
Szostak, M.; Sautier, B.; Procter, D. J.
Org. Lett. 2014, 16, 452.
Szostak, M.; Sautier, B.; Procter, D. J.
Org. Lett. 2014, 16, 452.
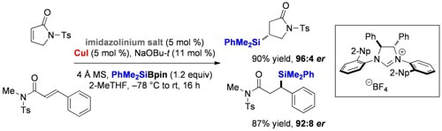
Cu(I)–NHC Catalyzed Asymmetric Silyl Transfer to Unsaturated Lactams and Amides
Pace, V.; Rae, J. P.; Procter, D. J.
Org. Lett. 2014, 16, 476.
Highlighted in Synfacts 2014, 10(4), 0400
Pace, V.; Rae, J. P.; Procter, D. J.
Org. Lett. 2014, 16, 476.
Highlighted in Synfacts 2014, 10(4), 0400
2013
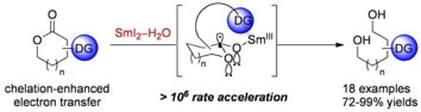
Substrate-Directable Electron Transfer Reactions. Dramatic Rate Enhancement in the Chemoselective Reduction of Cyclic Esters Using SmI2–H2O: Mechanism, Scope, and Synthetic Utility
Szostak, M.; Spain, M.; Choquette, K. A.; Flowers, II, R. A.; Procter, D. J.
J. Am. Chem. Soc. 2013, 135, 15702
Highlighted in Synform
Szostak, M.; Spain, M.; Choquette, K. A.; Flowers, II, R. A.; Procter, D. J.
J. Am. Chem. Soc. 2013, 135, 15702
Highlighted in Synform
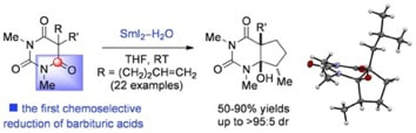
Selective Reduction of Barbituric Acids Using SmI2/H2O: Synthesis, Reactivity, and Structural Analysis of Tetrahedral Adducts
Szostak, M.; Sautier, B.; Spain, M.; Behlendorf, M.; Procter, D. J.
Angew. Chem. Int. Ed. 2013, 52, 12559
Szostak, M.; Sautier, B.; Spain, M.; Behlendorf, M.; Procter, D. J.
Angew. Chem. Int. Ed. 2013, 52, 12559
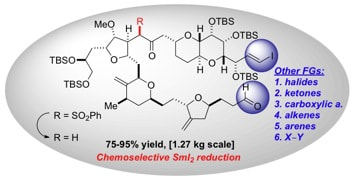
Recent advances in the chemoselective reduction of functional groups mediated by samarium(II) iodide: a single electron transfer approach
Szostak, M.; Spain, M.; Procter, D. J.
Chem. Soc. Rev. 2013, 42, 9155
Szostak, M.; Spain, M.; Procter, D. J.
Chem. Soc. Rev. 2013, 42, 9155
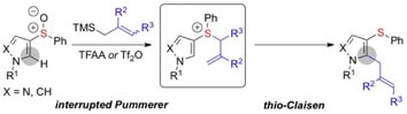
Nucleophilic ortho-Allylation of Pyrroles and Pyrazoles: An Accelerated Pummerer/Thio-Claisen Rearrangement Sequence
Eberhart, A. J.; Cicoira, C.; Procter, D. J.
Org. Lett. 2013, 15, 3994
Eberhart, A. J.; Cicoira, C.; Procter, D. J.
Org. Lett. 2013, 15, 3994
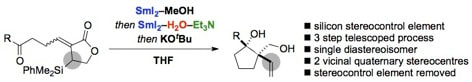
Development of an additive-controlled, SmI2-mediated stereoselective sequence: Telescoped spirocyclisation, lactone reduction and Peterson elimination
Sautier, B.; Collins, K. D.; Procter, D. J.
Beilstein J. Org. Chem. 2013, 9, 1443
Invited contribution to BJOC thematic series: "Organic Free Radical Chemistry", ed. Prof. Corey Stephenson
Sautier, B.; Collins, K. D.; Procter, D. J.
Beilstein J. Org. Chem. 2013, 9, 1443
Invited contribution to BJOC thematic series: "Organic Free Radical Chemistry", ed. Prof. Corey Stephenson
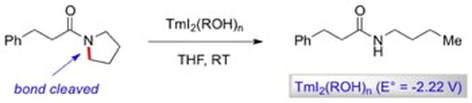
Uncovering the Importance of Proton Donors in TmI2-Promoted Electron Transfer: Facile C-N Bond Cleavage in Unactivated Amides
Szostak, M.; Spain, M.; Procter, D. J.
Angew. Chem. Int. Ed. 2013, 52, 7237
Highlighted in Synfacts 2013, 9(9), 1001
Organic Chemistry Highlights
Szostak, M.; Spain, M.; Procter, D. J.
Angew. Chem. Int. Ed. 2013, 52, 7237
Highlighted in Synfacts 2013, 9(9), 1001
Organic Chemistry Highlights
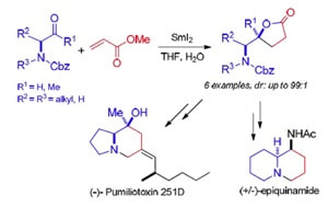
SmI2-mediated couplings of a-amino acid derivatives. Formal synthesis of (-)-pumiliotoxin 251D and (+/-)-epiquinamide
Pinho, V. D.; Procter, D. J.; Burtoloso, A. C. B.
Org. Lett. 2013, 15, 2434.
Pinho, V. D.; Procter, D. J.; Burtoloso, A. C. B.
Org. Lett. 2013, 15, 2434.
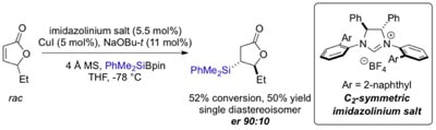
NHC-Cu(I) Catalysed Asymmetric Conjugate Silyl Transfer to Unstaurated Lactones: Application in Kinetic Resolution
Pace, V.; Rae, J. P.; Harb. H. Y.;Procter, D. J.
Chem. Commun. 2013, 49, 5150
Highlighted in Synfacts 2013, 9(9), 983
Pace, V.; Rae, J. P.; Harb. H. Y.;Procter, D. J.
Chem. Commun. 2013, 49, 5150
Highlighted in Synfacts 2013, 9(9), 983
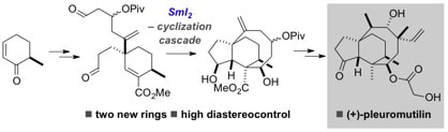
Total synthesis of (+)-pleuromutilin
Fazakerley, N. J.; Helm, M. D.; Procter, D. J.
Chem. -Eur. J. 2013, 19, 6718
Selected for Cover Art
Organic Chemistry Highlights
Fazakerley, N. J.; Helm, M. D.; Procter, D. J.
Chem. -Eur. J. 2013, 19, 6718
Selected for Cover Art
Organic Chemistry Highlights
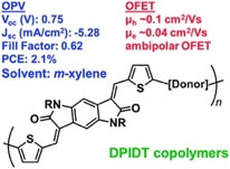
Dihydropyrroloindoledione-based copolymers for organic electronics
Rumer, J. W.; Dai, S-Y.; Levick, M.; Kim, Y.; Madec, M-B.; Ashraf, R. S.; Huang, Z.; Rossbauer, S.; Schroeder, B.; Biniek, L.; Watkins, S. E.; Anthopoulos, T. D.; Janssen, R. A. J.; Durrant, J. R.; Procter, D. J.; McCulloch, I.
J. Mater. Chem. C 2013, 1, 2711.
Rumer, J. W.; Dai, S-Y.; Levick, M.; Kim, Y.; Madec, M-B.; Ashraf, R. S.; Huang, Z.; Rossbauer, S.; Schroeder, B.; Biniek, L.; Watkins, S. E.; Anthopoulos, T. D.; Janssen, R. A. J.; Durrant, J. R.; Procter, D. J.; McCulloch, I.
J. Mater. Chem. C 2013, 1, 2711.
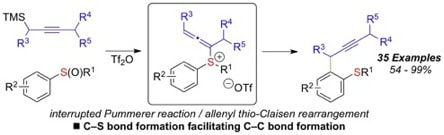
Nucleophilic ortho-propargylation of aryl sulfoxides: an interrrupted Pummerer/allenyl thio-Claisen rearrangement sequence
Eberhardt, A. J.; Procter, D. J.
Angew. Chem. Int. Ed. 2013, 52, 4008.
Eberhardt, A. J.; Procter, D. J.
Angew. Chem. Int. Ed. 2013, 52, 4008.
Synthesis of two dihydropyrroloindoledione-based copolymers for organic electronics
Rumer, J. W.; Dai, Sheng-Yao; Levick, M.; Biniek, L.; Procter, D. J.; McCulloch, I.
J. Polym. Sci. A Polym. Chem. 2013, 51, 1285.
Rumer, J. W.; Dai, Sheng-Yao; Levick, M.; Biniek, L.; Procter, D. J.; McCulloch, I.
J. Polym. Sci. A Polym. Chem. 2013, 51, 1285.
2012
Phase Tag-Assisted Synthesis of Benzo[b]carbazole End-Capped Oligothiophenes
Levick, M. T.; Coote, S. C.; Grace, I.; Lambert, C.; Turner, M. L.; Procter, D. J. Org. Lett. 2012, 14, 5744
Levick, M. T.; Coote, S. C.; Grace, I.; Lambert, C.; Turner, M. L.; Procter, D. J. Org. Lett. 2012, 14, 5744
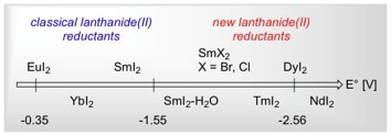
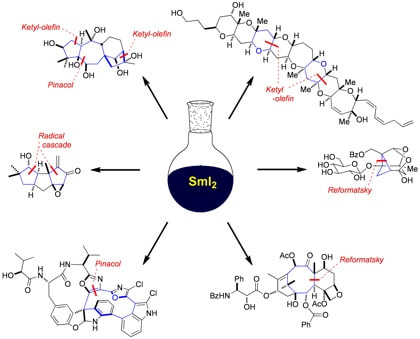
Beyond Samarium Diiodide: Vistas in Reductive Chemistry Mediated by Lanthanides(II)
Szostak, M.; Procter, D. J.
Angew. Chem. Int. Ed. 2012, 51, 9238.
Szostak, M.; Procter, D. J.
Angew. Chem. Int. Ed. 2012, 51, 9238.
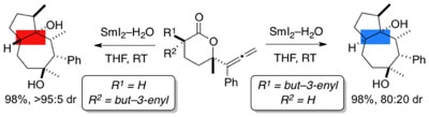
Lactone radical cyclizations and cyclization cascades mediated by SmI2–H2O
Parmar, D.; Matsubara, H.; Price, K.; Spain, M.; Procter, D. J.
J. Am. Chem. Soc. 2012, 134, 12751.
Parmar, D.; Matsubara, H.; Price, K.; Spain, M.; Procter, D. J.
J. Am. Chem. Soc. 2012, 134, 12751.

Recent advances in the chemistry of SmI2–H2O
Sautier, B.; Procter, D. J.
Chimia 2012, 66, 399
Invited contribution to Special Issue. Selected for Cover Art
Sautier, B.; Procter, D. J.
Chimia 2012, 66, 399
Invited contribution to Special Issue. Selected for Cover Art

A General Electron Transfer Reduction of Lactones Using SmI2–H2O
Szostak, M.; Collins, K. D.; Fazakerley, N. J.; Spain, M.; Procter, D. J.
Org. Biomol. Chem. 2012, 10, 5820
Invited contribution to "Organic & Biomolecular Chemistry 10th Anniversary" Collection.
Szostak, M.; Collins, K. D.; Fazakerley, N. J.; Spain, M.; Procter, D. J.
Org. Biomol. Chem. 2012, 10, 5820
Invited contribution to "Organic & Biomolecular Chemistry 10th Anniversary" Collection.

Selective synthesis of 3-hydroxy acids from Meldrum’s acids using SmI2-H2O
Szostak, M.; Spain, M.; Procter, D. J.
Nat. Protocol 2012, 7, 970.
Szostak, M.; Spain, M.; Procter, D. J.
Nat. Protocol 2012, 7, 970.
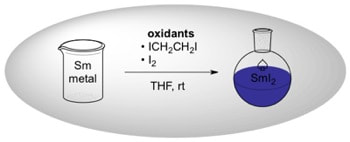
Preparation of Samarium(II) Iodide: Quantitative Evaluation of the Effect of Water, Oxygen, and Peroxide Content, Preparative Methods, and the Activation of Samarium Metal
Szostak, M.; Spain, M.; Procter, D. J.
J. Org. Chem. 2012, 77, 3049.
Szostak, M.; Spain, M.; Procter, D. J.
J. Org. Chem. 2012, 77, 3049.

Electron transfer reduction of carboxylic acids using SmI2-H2O-Et3N
Szostak, M.; Spain, M.; Procter, D. J.
Org. Lett. 2012, 14, 840.
Szostak, M.; Spain, M.; Procter, D. J.
Org. Lett. 2012, 14, 840.

SmI2-Mediated Carbonyl-Alkene Couplings for the Synthesis of Small Carbocyclic Rings
Harb, Y. H.; Procter, D. J.
Synlett 2012, 23, 6.
Harb, Y. H.; Procter, D. J.
Synlett 2012, 23, 6.
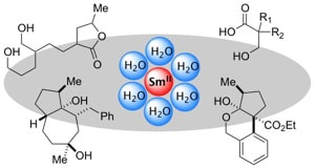
Selective Reductive Transformations Using Samarium Diiodide-Water
Szostak, M.; Spain, M.; Parmar, D.; Procter, D. J.
Chem Commun. 2012, 48, 330.
Szostak, M.; Spain, M.; Parmar, D.; Procter, D. J.
Chem Commun. 2012, 48, 330.
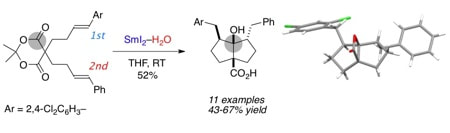
Radical Cyclization Cascades of Unsaturated Meldrum's Acid Derivatives
Sautier, B.; Lyons, S. E.; Webb, M. R.; Procter, D. J.
Org. Lett. 2012, 14, 146.
Sautier, B.; Lyons, S. E.; Webb, M. R.; Procter, D. J.
Org. Lett. 2012, 14, 146.
Organic Synthesis Using Samarium Diiodide
Coote, S. C.; Flowers II, R. A.; Skrydstrup, T.; Procter, D. J.
in Encyclopedia of Radicals in Chemistry, Biology and Materials 2012, John Wiley & Sons, Ltd.
Coote, S. C.; Flowers II, R. A.; Skrydstrup, T.; Procter, D. J.
in Encyclopedia of Radicals in Chemistry, Biology and Materials 2012, John Wiley & Sons, Ltd.
2011

Nucleophilic Ortho Allylation of Aryl and
Heteroaryl Sulfoxides
Eberhart, A. J.; Imbriglio, J. E.; Procter, D. J.
Org. Lett. 2011, 13, 5882
Heteroaryl Sulfoxides
Eberhart, A. J.; Imbriglio, J. E.; Procter, D. J.
Org. Lett. 2011, 13, 5882

Synthesis of the ABH rings of ecteinascidin 597 using a connective Pummerer-type cyclisation
Smith, L. H. S.; Nguyen, T. T.; Sneddon, H. F.; Procter, D. J.
Chem. Commun. 2011, 47, 10821.
Smith, L. H. S.; Nguyen, T. T.; Sneddon, H. F.; Procter, D. J.
Chem. Commun. 2011, 47, 10821.
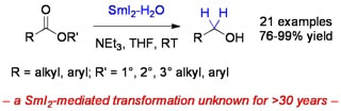
Electron transfer reduction of unactivated esters using SmI2-H2O
Szostak, M.; Spain, M.; Procter, D. J.
Chem. Commun. 2011, 47, 10254.
Szostak, M.; Spain, M.; Procter, D. J.
Chem. Commun. 2011, 47, 10254.
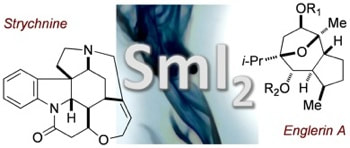
Concise Syntheses of Strychnine and Englerin A: the Power of Reductive Cyclizations Triggered by Samarium Iodide for the Rapid Assembly of Old and New Targets
Szostak, M.; Procter, D. J.
Angew. Chem. Int. Ed. 2011, 50, 7737.
Szostak, M.; Procter, D. J.
Angew. Chem. Int. Ed. 2011, 50, 7737.
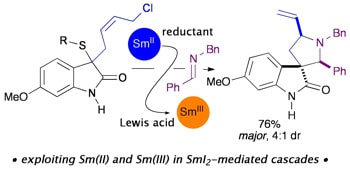
Exploiting Sm(II) and Sm(III) in SmI2-initiated reaction cascades: application in a tag removal-cyclisation approach to spirooxindole scaffolds
Coote, S. C.; Quenum S.; Procter , D. J.
Org. Biomol. Chem. 2011, 9, 5104.
Coote, S. C.; Quenum S.; Procter , D. J.
Org. Biomol. Chem. 2011, 9, 5104.
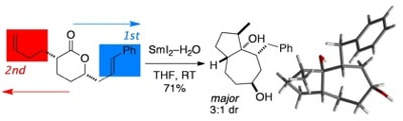
Reductive Cyclization Cascades of Lactones Using SmI2–H2O
Parmar, D.; Price, K.; Spain, M.; Matsubara, H.;Bradley, P. A.; Procter, D. J.
J. Am. Chem. Soc. 2011, 133, 2418.
Parmar, D.; Price, K.; Spain, M.; Matsubara, H.;Bradley, P. A.; Procter, D. J.
J. Am. Chem. Soc. 2011, 133, 2418.
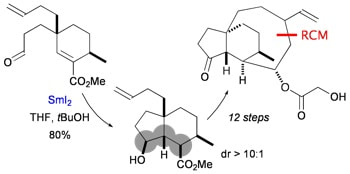
A stereoselective, Sm(II)–mediated approach to decorated cis–hydrindanes: synthetic studies on faurinone and pleuromutilin
Findley, T. J. K.; Sucunza, D.; Miller, L. C.; Helm, M. D.; Helliwell, M.; Davies, D. T.; Procter, D. J.
Org. Biomol. Chem. 2011, 9, 2433
Invited contribution to the Athel Beckwith Special issue.
Findley, T. J. K.; Sucunza, D.; Miller, L. C.; Helm, M. D.; Helliwell, M.; Davies, D. T.; Procter, D. J.
Org. Biomol. Chem. 2011, 9, 2433
Invited contribution to the Athel Beckwith Special issue.
2010
Product Class 17: Acyclic Hemiacetals, Lactols, and Carbonyl Hydrates
Coote, S. C.; Smith, L. H. S.; Procter, D. J.
Science of Synthesis Section 29.17, Thieme, Volume Update Editor, Procter, D. J. 2010, 417.
Invited Volume Update Editor
Coote, S. C.; Smith, L. H. S.; Procter, D. J.
Science of Synthesis Section 29.17, Thieme, Volume Update Editor, Procter, D. J. 2010, 417.
Invited Volume Update Editor
Product Class 18: 1,1–Diacyloxy compounds
Smith, L. H. S.; Coote, S. C.; Procter, D. J.
Science of Synthesis Section 29.18, Thieme, Volume Update Editor, Procter, D. J. 2010, 475.
Invited Volume Update Editor
Smith, L. H. S.; Coote, S. C.; Procter, D. J.
Science of Synthesis Section 29.18, Thieme, Volume Update Editor, Procter, D. J. 2010, 475.
Invited Volume Update Editor
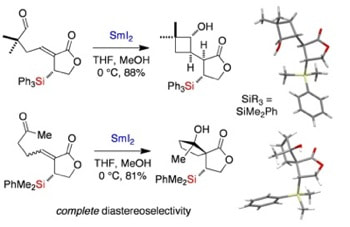
SmI2–mediated radical cyclizations directed by a C−Si bond
Harb, H. Y.; Collins, K. D.; Garcia Altur, J. V.; Bowker, S.; Campbell, L.; Procter, D. J. Org. Lett. 2010, 12, 5446.
Harb, H. Y.; Collins, K. D.; Garcia Altur, J. V.; Bowker, S.; Campbell, L.; Procter, D. J. Org. Lett. 2010, 12, 5446.
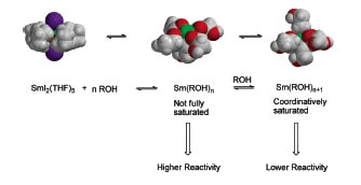
Dynamic Ligand Exchange in Reactions of Samarium Diiodide
Sadasivam, D. V.; Teprovich Jr, J. A.; Procter, D. J.; Flowers II, R. A.
Org. Lett. 2010, 12, 4140.
Sadasivam, D. V.; Teprovich Jr, J. A.; Procter, D. J.; Flowers II, R. A.
Org. Lett. 2010, 12, 4140.
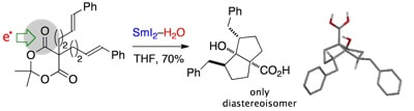
Selective reductions of cyclic 1,3-diesters using SmI2 and H2O
Collins, K. D.; Oliveira, J. M.; Guazzelli, G.; Sautier, B.; De Grazia, S.; Matsubara, H.; Helliwell, M.; Procter, D. J.
Chem. –Eur. J. 2010, 16, 10240.
Collins, K. D.; Oliveira, J. M.; Guazzelli, G.; Sautier, B.; De Grazia, S.; Matsubara, H.; Helliwell, M.; Procter, D. J.
Chem. –Eur. J. 2010, 16, 10240.
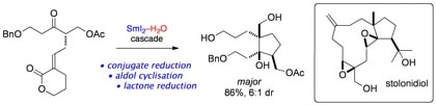
A Stereoselective Cyclization Cascade Mediated by SmI2-H2O: Synthetic Studies towards Stolonidiol
Baker, T. M.; Sloan, L. A.; Choudhury, L. H.; Murai, M.; Procter, D. J.
Tetrahedron: Asymmetry 2010, 21, 1246.
Invited contribution to the Henri Kagan Special issue.
Baker, T. M.; Sloan, L. A.; Choudhury, L. H.; Murai, M.; Procter, D. J.
Tetrahedron: Asymmetry 2010, 21, 1246.
Invited contribution to the Henri Kagan Special issue.
Beyond the Pummerer Reaction: Recent Developments in Thionium Ion Chemistry
Smith, L. H. S.; Coote, S. C.; Sneddon, H. F.; Procter, D. J.
Angew. Chem. Int. Ed. 2010, 49, 5832
Smith, L. H. S.; Coote, S. C.; Sneddon, H. F.; Procter, D. J.
Angew. Chem. Int. Ed. 2010, 49, 5832
2009

Organic Synthesis using Samarium Diiodide: A Practical Guide
Procter, D. J.; Flowers, R. A., II; Skrydstrup, T.
RSC Publishing 2009.
Procter, D. J.; Flowers, R. A., II; Skrydstrup, T.
RSC Publishing 2009.
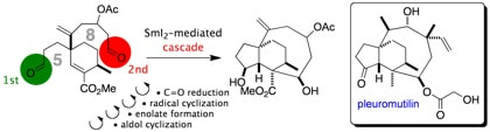
A dialdehyde cyclization cascade in an approach to pleuromutilin
Helm, M. D.; Da Silva, M.; Sucunza, D.; Findley, T. J. K.; Procter, D. J.
Angew. Chem. Int. Ed. 2009, 48, 9315.
Helm, M. D.; Da Silva, M.; Sucunza, D.; Findley, T. J. K.; Procter, D. J.
Angew. Chem. Int. Ed. 2009, 48, 9315.
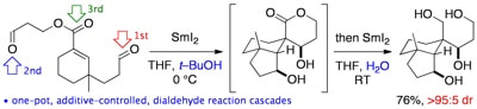
SmI2–mediated dialdehyde ‘radical then aldol’ cyclization cascades: a feasibility study
Helm, M. D.; Da Silva, M.; Sucunza, D.; Helliwell,M.; Procter, D. J.
Tetrahedron, 2009, 65, 10816
Special issue, "Electron transfer reagents in organic synthesis". Edited by D. J. Procter and R. A. Flowers, II
Helm, M. D.; Da Silva, M.; Sucunza, D.; Helliwell,M.; Procter, D. J.
Tetrahedron, 2009, 65, 10816
Special issue, "Electron transfer reagents in organic synthesis". Edited by D. J. Procter and R. A. Flowers, II

Studies on the mechanism, selectivity and synthetic utility of lactone reduction using SmI2 and H2O
Parmar, D.; Duffy, L. A.; Sadasivam, D. V.; Matsubara, H.; Bradley, P. A.; Flowers II, R. A.; Procter, D. J.
J. Am. Chem. Soc., 2009, 131, 15467
Parmar, D.; Duffy, L. A.; Sadasivam, D. V.; Matsubara, H.; Bradley, P. A.; Flowers II, R. A.; Procter, D. J.
J. Am. Chem. Soc., 2009, 131, 15467
Linker units cleaved by radical processes
Guazzelli, G.; Miller, M.; Procter, D. J.
In Linker Strategies In Solid–Phase Organic Synthesis, Ed. Peter J. H. Scott, Wiley, 2009, Chapter 15, 419.
Invited contribution
Guazzelli, G.; Miller, M.; Procter, D. J.
In Linker Strategies In Solid–Phase Organic Synthesis, Ed. Peter J. H. Scott, Wiley, 2009, Chapter 15, 419.
Invited contribution
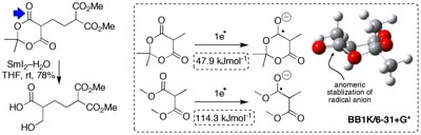
Selective reductions of cyclic 1,3–diesters using SmI2 and H2O
Guazzelli, G.; De Grazia, S.; Collins, K. D.; Matsubara, H.; Spain, M.; Procter, D. J.
J. Am. Chem. Soc., 2009, 131, 7214.
Guazzelli, G.; De Grazia, S.; Collins, K. D.; Matsubara, H.; Spain, M.; Procter, D. J.
J. Am. Chem. Soc., 2009, 131, 7214.
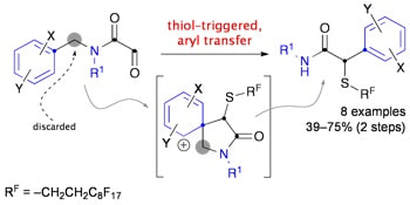
Intramolecular aryl transfer to thionium ions in an approach to α-arylacetamides
Ovens, C.;, Vogel, J. C.; Martin, N. G.; Procter, D. J.
Chem. Commun., 2009, 3101
Ovens, C.;, Vogel, J. C.; Martin, N. G.; Procter, D. J.
Chem. Commun., 2009, 3101
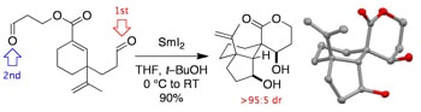
SmI2–mediated dialdehyde cyclization cascades
Helm, M. D.; Sucunza, D.; Da Silva, M.; Helliwell, M.; Procter, D. J.
Tetrahedron Lett., 2009, 50, 3224
Invited contribution to the 50th Anniversary special issue
Helm, M. D.; Sucunza, D.; Da Silva, M.; Helliwell, M.; Procter, D. J.
Tetrahedron Lett., 2009, 50, 3224
Invited contribution to the 50th Anniversary special issue
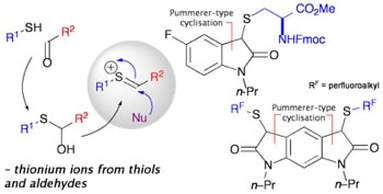
Formation of N–heterocycles by the reaction of thiols with glyoxamides: Exploring a connective Pummerer-type cyclisation
Miller, M.; Vogel, J.; Tsang, W.; Merrit, A.; Procter, D. J.
Org. Biomol. Chem., 2009, 7, 589
Miller, M.; Vogel, J.; Tsang, W.; Merrit, A.; Procter, D. J.
Org. Biomol. Chem., 2009, 7, 589
2008
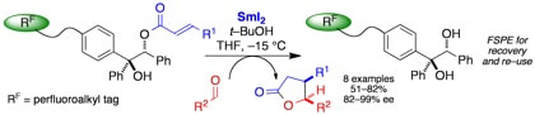
An asymmetric, SmI2–mediated approach to γ–butryolactones using a new, fluorous-tagged auxiliary
Vogel, J.; Butler, R.; Procter. D. J.
Tetrahedron, 2008, 64, 11876
Invited contribution to a special issue “Modern Radical Methodology In Organic Synthesis”
Vogel, J.; Butler, R.; Procter. D. J.
Tetrahedron, 2008, 64, 11876
Invited contribution to a special issue “Modern Radical Methodology In Organic Synthesis”
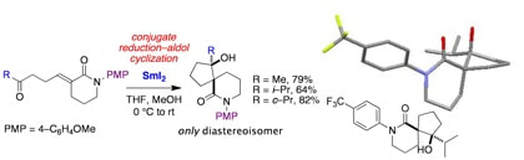
A samarium(II)–mediated, stereoselective cyclization for the synthesis of spirocyclic lactams
Guazzelli, G.; Duffy, L. A. Procter, D. J.
Org. Lett., 2008, 10, 4291
Guazzelli, G.; Duffy, L. A. Procter, D. J.
Org. Lett., 2008, 10, 4291
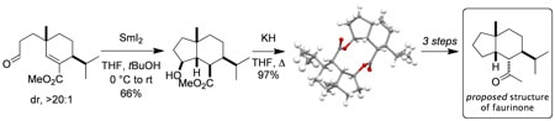
A flexible, stereoselective approach to the decorated cis–hydrindane skeleton: synthesis of the proposed structure of faurinone
Findley, T. J. K.; Sucunza, D.; Miller, L. C.; Davies, D. T.; Procter, D. J.
Chem. Eur. J., 2008, 14, 6862
Findley, T. J. K.; Sucunza, D.; Miller, L. C.; Davies, D. T.; Procter, D. J.
Chem. Eur. J., 2008, 14, 6862
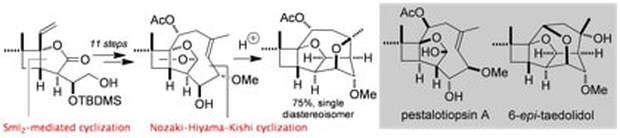
Synthesis and reactions of the pestalotiopsin skeleton
Baker, T. M.; Edmonds, D. J.; Hamilton, D.; O’Brien, C. J.; Procter, D. J.
Angew. Chem. Int. Ed., 2008, 47, 5631
Baker, T. M.; Edmonds, D. J.; Hamilton, D.; O’Brien, C. J.; Procter, D. J.
Angew. Chem. Int. Ed., 2008, 47, 5631
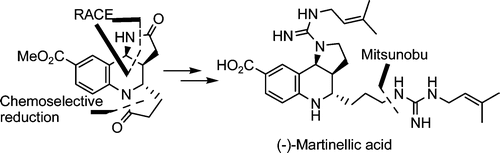
Total Synthesis of (-)-Martinellic Acid via Radical Addition-Cyclization-Elimination Reaction
Shirai, A.; Miyata, O.; Tohnai, N.; Miyata, M.; Naito, T.; Procter, D. J.; Sucunza, D.
J. Org. Chem. ,2008, 73, 4464.
Shirai, A.; Miyata, O.; Tohnai, N.; Miyata, M.; Naito, T.; Procter, D. J.; Sucunza, D.
J. Org. Chem. ,2008, 73, 4464.
Methanolysis of Tetraphenylborate (BPh4-) as a reaction unit in halotris(2,4-pentadianato) complexes of Zr(IV) and Hf(IV)
Mlondo, S. N.; O’Brien, P.; Thomas, P. J.; Helliwell, M.; Raftery, J.; Procter, D. J.
Chem. Commun., 2008, 2456.
Mlondo, S. N.; O’Brien, P.; Thomas, P. J.; Helliwell, M.; Raftery, J.; Procter, D. J.
Chem. Commun., 2008, 2456.
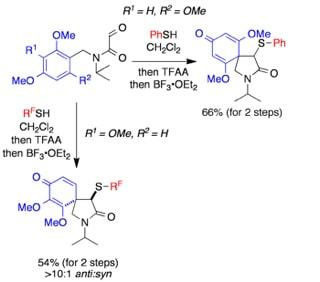
A dearomatizing, thionium ion cyclization for the synthesis of functionalized, azaspirocyclic cyclohexadienones
Ovens, C.; Martin, N. G.; Procter, D. J.
Org. Lett., 2008, 10, 1441
Ovens, C.; Martin, N. G.; Procter, D. J.
Org. Lett., 2008, 10, 1441
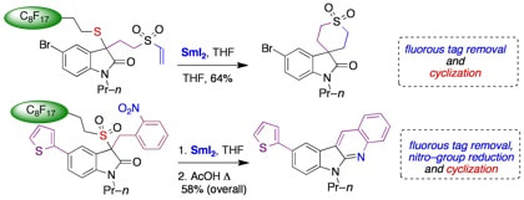
Samarium(II)–mediated linker cleavage–cyclization in fluorous synthesis: reactions of samarium enolates
James, K. M.; Willetts, N.; Procter, D. J.
Org. Lett., 2008, 10, 1203
James, K. M.; Willetts, N.; Procter, D. J.
Org. Lett., 2008, 10, 1203

A Ring Size-Selective Reduction of Lactones Using SmI2 and H2O
Duffy, L. A.; Matsubara, H.; Procter, D. J.
J. Am. Chem. Soc., 2008, 130, 1136
Highlighted in Org. Proc. Res. Dev., 2008, 12, 358-368.
Duffy, L. A.; Matsubara, H.; Procter, D. J.
J. Am. Chem. Soc., 2008, 130, 1136
Highlighted in Org. Proc. Res. Dev., 2008, 12, 358-368.
Samarium enolates and their application in organic synthesis
Rudkin, I. M.; Miller, L. C.; Procter, D. J.
Organomet. Chem., 2008, 34, 19
Rudkin, I. M.; Miller, L. C.; Procter, D. J.
Organomet. Chem., 2008, 34, 19
pre-2008

Application of a Sm(II)-mediated spirocyclization in an asymmetric approach to the cyclopentanol motif of stolonidiol
Sloan, L. A.; Baker, T.; Macdonald, S. J. F.; Procter, D. J.
Synlett, 2007, 3155
Sloan, L. A.; Baker, T.; Macdonald, S. J. F.; Procter, D. J.
Synlett, 2007, 3155

Synthesis and evaluation of a new polymer–supported pseudoephedrine auxiliary for asymmetric alkylations on solid phase
McGhee, A. M.; Kizirian, J–C.; Procter, D. J.
Org. Biomol. Chem., 2007, 5, 1021
McGhee, A. M.; Kizirian, J–C.; Procter, D. J.
Org. Biomol. Chem., 2007, 5, 1021
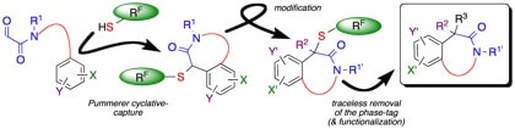
A Fluorous, Pummerer Cyclative–Capture Strategy for the Synthesis of N–Heterocycles
McAllister, L. A.; McCormick, R. A.; James, K. M.; Brand, S.; Willetts, N.; Procter, D. J.
Chem. Eur J., 2007, 13, 1032
McAllister, L. A.; McCormick, R. A.; James, K. M.; Brand, S.; Willetts, N.; Procter, D. J.
Chem. Eur J., 2007, 13, 1032
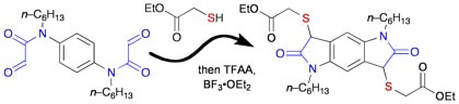
Exploring a new, connective Pummerer reaction: formation of oxindoles by the reaction of thiols with glyoxamides
Miller, M.; Tsang, W.; Merritt, A.; Procter, D. J.
Chem. Commun., 2007, 498
Miller, M.; Tsang, W.; Merritt, A.; Procter, D. J.
Chem. Commun., 2007, 498
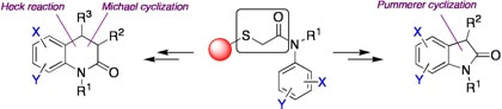
Solid Phase Approaches to N-heterocycles using a sulfur linker cleaved using SmI2
McAllister, L. A.; Turner, K. L.; Brand, S.; Stefaniak, M.; Procter, D. J.
J. Org. Chem., 2006, 71, 6497
McAllister, L. A.; Turner, K. L.; Brand, S.; Stefaniak, M.; Procter, D. J.
J. Org. Chem., 2006, 71, 6497
Lanthanide Reagents in Solid Phase Synthesis
Sloan, L. A.; Procter, D. J.
Chem. Soc. Rev., 2006, 35, 1221
Sloan, L. A.; Procter, D. J.
Chem. Soc. Rev., 2006, 35, 1221
Developing New Cleavage Strategies in a Fluorous Phase Pummerer Cyclative–Capture Approach for the Synthesis of N–Heterocycles
McCormick, R. A.; James, K. M.; Willetts, N.; Procter, D. J.
QSAR Comb. Sci., 2006, 25, 709
Invited contribution to a special issue on fluorous chemistry
McCormick, R. A.; James, K. M.; Willetts, N.; Procter, D. J.
QSAR Comb. Sci., 2006, 25, 709
Invited contribution to a special issue on fluorous chemistry
Radical Chemistry on Solid Support
McGhee, A. M. and Procter, D. J.
Top. Curr. Chem., 2006, 264, 93
Invited contribution to a special issue on radical chemistry
McGhee, A. M. and Procter, D. J.
Top. Curr. Chem., 2006, 264, 93
Invited contribution to a special issue on radical chemistry

A solid phase approach to tetrahydroquinolones using a sulfur linker cleaved by SmI2
Turner, K. L.; Baker, T. M.; Islam, S.; Procter, D. J.; Stefaniak, M.
Org. Lett., 2006, 8, 329
Turner, K. L.; Baker, T. M.; Islam, S.; Procter, D. J.; Stefaniak, M.
Org. Lett., 2006, 8, 329
Sulfide and selenide–base linkers in phase tag–assisted synthesis
McAllister, L. A.; McCormick, R. A.; Procter, D. J.
Tetrahedron, 2005, 61, 11527
McAllister, L. A.; McCormick, R. A.; Procter, D. J.
Tetrahedron, 2005, 61, 11527
Evaluation of a new linker system cleaved using samarium(II) iodide. Application in the solid phase synthesis of carbonyl compounds
F. McKerlie, F.; Rudkin, I. M.; Wynne, G.; and Procter, D. J.
Org. Biomol. Chem., 2005, 3, 2805
F. McKerlie, F.; Rudkin, I. M.; Wynne, G.; and Procter, D. J.
Org. Biomol. Chem., 2005, 3, 2805
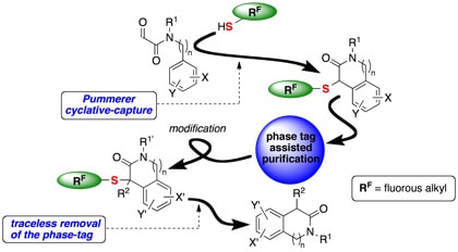
A fluorous-phase Pummerer cyclative-capture strategy for the synthesis of nitrogen heterocycles
McAllister, L. A.; McCormick, R. A.; Brand, S.; Procter, D. J.
Angew. Chem. Int. Ed., 2005, 44, 452
McAllister, L. A.; McCormick, R. A.; Brand, S.; Procter, D. J.
Angew. Chem. Int. Ed., 2005, 44, 452

The samarium(II)-mediated intermolecular couplings of ketones and β-alkoxyacrylates: A short asymmetric synthesis of an antifungal γ-butyrolactone
Kerrigan, N. J.; Upadhyay, T.; Procter, D. J.
Tetrahedron Lett., 2004, 45, 9087
Kerrigan, N. J.; Upadhyay, T.; Procter, D. J.
Tetrahedron Lett., 2004, 45, 9087

Development of a solid-phase 'asymmetric resin-capture - release' process: Application of an ephedrine chiral resin in an approach to γ-butyrolactones
Kerrigan, N. J.; Hutchison, P. C.; Heightman, T. D.; Procter, D. J.
Org. Biomol. Chem., 2004, 2476.
Kerrigan, N. J.; Hutchison, P. C.; Heightman, T. D.; Procter, D. J.
Org. Biomol. Chem., 2004, 2476.
Samarium(II) iodide-mediated cyclizations in natural product synthesis
Edmonds, D. J.; Johnston, D.; Procter, D. J.
Chem. Rev., 2004, 104, 3371
Edmonds, D. J.; Johnston, D.; Procter, D. J.
Chem. Rev., 2004, 104, 3371
Application of a recyclable pseudoephedrine resin in asymmetric alkylations on solid phase
Hutchison, P. C.; Heightman, T. D.; Procter, D. J.
J. Org. Chem., 2004, 790
Hutchison, P. C.; Heightman, T. D.; Procter, D. J.
J. Org. Chem., 2004, 790
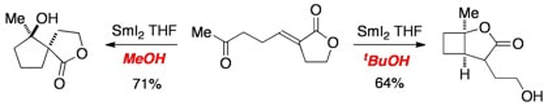
Switching between novel samarium(II)-mediated cyclizations by a simple change in alcohol cosolvent
Hutton, T. K.; Muir, K. W.; Procter, D. J.
Org. Lett., 2003, 5, 4811
Hutton, T. K.; Muir, K. W.; Procter, D. J.
Org. Lett., 2003, 5, 4811
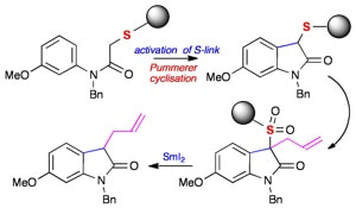
The first Pummerer cyclisations on solid phase. Convenient construction of oxindoles enabled by a sulfur-link to resin
McAllister, L. A.; Brand, S.; de Gentile, R.; Procter, D. J.
Chem. Commun., 2003, 2380
McAllister, L. A.; Brand, S.; de Gentile, R.; Procter, D. J.
Chem. Commun., 2003, 2380
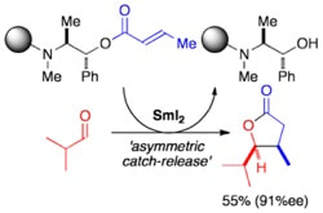
Application of an ephedrine chiral linker in a solid-phase, 'asymmetric catch-release' approach to γ-butyrolactones
Kerrigan, N. J.; Hutchison, P. C.; Heightman, T. D.; Procter, D. J.
Chem. Commun., 2003, 1402
Kerrigan, N. J.; Hutchison, P. C.; Heightman, T. D.; Procter, D. J.
Chem. Commun., 2003, 1402
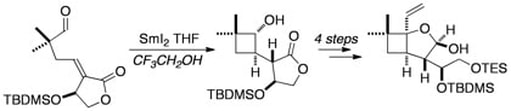
The Remarkable Effect of co-solvent on a Samarium(II)-mediated 4-exo-trig Cyclisation: Further Synthetic Studies on Pestalotiopsin A
Edmonds, D. J.; Muir, K. W.; Procter, D. J.
J. Org. Chem., 2003, 68 ,3190.
Edmonds, D. J.; Muir, K. W.; Procter, D. J.
J. Org. Chem., 2003, 68 ,3190.
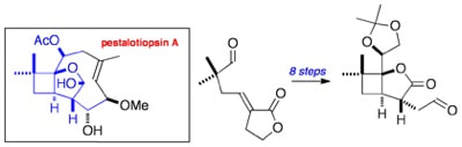
The first synthetic studies on pestalotiopsin A. A stereocontrolled approach to the functionalised bicyclic core
Johnston, D.; Couché, E.; Edmonds, D. J.; Muir, K.; Procter, D. J.
Org. Biomol. Chem., 2003, 1, 328
Johnston, D.; Couché, E.; Edmonds, D. J.; Muir, K.; Procter, D. J.
Org. Biomol. Chem., 2003, 1, 328
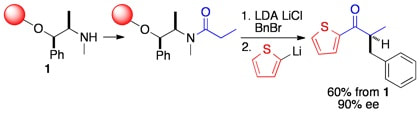
Evaluation of a pseudoephedrine linker for asymmetric alkylations on solid phase
Hutchison, P. C.; Heightman, T. D.; Procter, D. J.
Org. Lett., 2002, 4, 4583
Hutchison, P. C.; Heightman, T. D.; Procter, D. J.
Org. Lett., 2002, 4, 4583
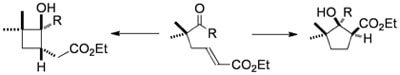
Samarium(II)-Mediated Reactions of γ,δ-Unsaturated Ketones. Cyclization and Fragmentation Processes
Hutton, T. K.; Muir, K.; Procter, D. J.
Org. Lett., 2002, 4, 2345
Hutton, T. K.; Muir, K.; Procter, D. J.
Org. Lett., 2002, 4, 2345
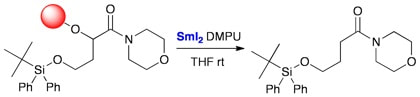
Reduction of a-aryloxy carbonyl compounds with samarium(II) iodide. A new traceless linker for the solid phase synthesis of carbonyl compounds
McKerlie, F.; Procter, D. J.; Wynne, G.
Chem. Commun., 2002, 584
McKerlie, F.; Procter, D. J.; Wynne, G.
Chem. Commun., 2002, 584
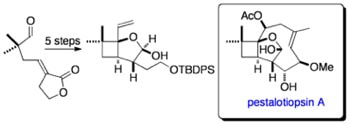
Samarium(II)-Mediated 4-exo-trig cyclization. A Stereocontrolled Approach to the Core of Pestalotiopsin A
Johnston, D.; Francon, N.; Edmonds, D. J.; Procter, D. J.
Org. Lett., 2001, 3, 2001
Johnston, D.; Francon, N.; Edmonds, D. J.; Procter, D. J.
Org. Lett., 2001, 3, 2001
The synthesis of thiols, selenols, sulfides, selenides, sulfoxides, selenoxides, sulfones and selenones
Procter, D. J.
J. Chem. Soc., Perkin Trans. 1, 2001, 335
Procter, D. J.
J. Chem. Soc., Perkin Trans. 1, 2001, 335
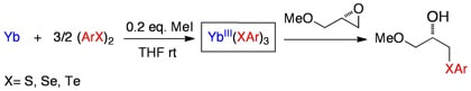
Convenient preparation of ytterbium(III) chalcogenolate complexes by insertion of ytterbium into chalcogen-chalcogen bonds. Application in the ring-opening of epoxides
Dowsland, J.; McKerlie, F.; Procter, D. J.
Tetrahedron Lett., 2000, 41, 4923
Dowsland, J.; McKerlie, F.; Procter, D. J.
Tetrahedron Lett., 2000, 41, 4923
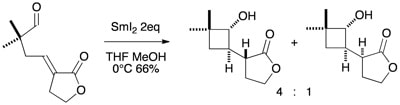
Samarium(II)-mediated 4-exo-trig cyclisations of unsaturated aldehydes. A stereoselective approach to functionalised cyclobutanols
Johnston, D.; McCusker, C. F.; Muir, K.; Procter, D. J.
J. Chem. Soc., Perkin Trans. 1, 2000, 681
Johnston, D.; McCusker, C. F.; Muir, K.; Procter, D. J.
J. Chem. Soc., Perkin Trans. 1, 2000, 681
The synthesis of thiols, selenols, sulfides, selenides, sulfoxides, selenoxides, sulfones and selenones
Procter, D. J.
J. Chem. Soc., Perkin Trans. 1, 2000, 835.
Procter, D. J.
J. Chem. Soc., Perkin Trans. 1, 2000, 835.
The synthesis of thiols, selenols, sulfides, selenides, sulfoxides, selenoxides, sulfones and selenones
Procter, D. J.
J. Chem. Soc., Perkin Trans. 1, 1999, 641
Procter, D. J.
J. Chem. Soc., Perkin Trans. 1, 1999, 641
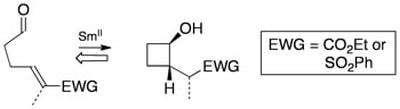
Samarium(II)-Mediated 4-exo trig Ketyl-Olefin Cyclisation of Unsaturated Aldehydes. A General, Stereoselective Synthesis of Functionalised Cyclobutanols
Johnston, D.; McCusker, C. M.; Procter, D. J.
Tetrahedron Lett. 1999 , 40, 4913.
Johnston, D.; McCusker, C. M.; Procter, D. J.
Tetrahedron Lett. 1999 , 40, 4913.